

PNAS | Researchers from the Ultrasensitive Magnetic Resonance Research Group Make Significant Progress in mRNA Vaccine Delivery Research
Recently, the ultra-sensitive magnetic resonance research team at the Innovation Academy for Precision Measurement Science and Technology (APM) has made important progress in mRNA vaccine delivery research. The team developed a novel lipid nanoparticle system with an intrinsic imaging capability that enables both efficient mRNA delivery and real-time, non-invasive in vivo tracking., This system provides a powerful tool for systematically elucidating the dynamic relationships among mRNA delivery, protein expression, and immune activation. The study has been published in the Proceedings of the National Academy of Sciences of the United States of America (PNAS).
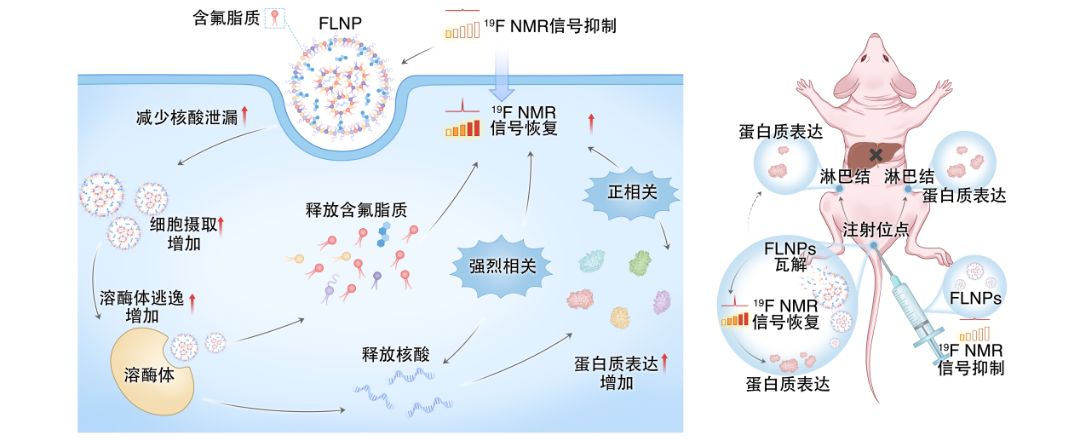
mRNA molecules are intrinsically unstable and susceptible to rapid degradation in vivo. necessitating the use of lipid nanoparticles (LNPs) as delivery vehicles. However, traditional LNPs face two limitations: their in vivo fate is difficult to determine; and a substantial fraction of the administered dose undergoes non-specific hepatic uptake. This off-target accumulation reduces delivery efficiency to intended tissues and raises potential safety concerns, thereby constraining both mechanistic studies and translational applications.
To overcome these challenges, the research team led by Xin Zhou introduced fluorinated structural motifs into LNPs, generating a new class of fluorinated lipid nanoparticles (FLNPs). The incorporated fluorine serves as a highly specific 19F magnetic resonance imaging (MRI) tracer. Because endogenous 19F background signals in the body are negligible, this strategy enables clear and quantitative visualization of nanocarrier biodistribution in vivo. Experimental results demonstrate that FLNPs maintain mRNA expression efficiency comparable to that of clinically used LNPs, while reducing non-specific liver accumulation by up to 94.6%. Importantly, this system allows continuous in vivo monitoring of nanocarrier distribution, mRNA release, and the spatiotemporal dynamics of antigen expression—capabilities that were previously difficult to achieve.
Building on this imaging platform, the team integrated FLNP tracking with immunological analyses to establish a coherent in vivo sequence linking nanocarrier localization, antigen expression, and immune cell behavior. The results show that immune activation is initiated locally at the injection site, followed by the migration of antigen-presenting cells to draining lymph nodes, thereby completing the early and essential steps of immune response initiation.
By addressing the central question of how mRNA vaccines function in vivo from both dynamic and mechanistic perspectives, this study provides direct experimental evidence for the spatiotemporal processes underlying immune activation. The FLNP platform, which combines precise delivery with non-invasive real-time tracking, offers a robust technological foundation for the rational design, safety assessment, and efficacy monitoring of next-generation mRNA vaccines and nucleic acid therapeutics.
The article, entitled "Fluorinated Lipid Nanoparticles Enable Real-Time Tracking of mRNA Delivery and Uncover Spatiotemporal Mechanisms of Immune Activation", lists Kairu Xie and Lijun Zhu as co-first authors, with Xin Zhou and Daiqin Chen as co-corresponding authors.
This work was supported by the Ministry of Science and Technology of China, the National Natural Science Foundation of China, and the Chinese Academy of Sciences, among other funding agencies.

Innovation Academy for Precision Measurement Science and Technology, CAS.
West No.30 Xiao Hong Shan, Wuhan 430071 China
Tel:+86-27-8719-8631 Fax:+86-27-8719-9291
Email:hanyeqing@wipm.ac.cn
鄂ICP备15017570号-1